
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Complications
- Switching from Conventional Fibrates to Pemafibrate Has Beneficial Effects on the Renal Function of Diabetic Subjects with Chronic Kidney Disease
- Rimi Izumihara, Hiroshi Nomoto, Kenichi Kito, Yuki Yamauchi, Kazuno Omori, Yui Shibayama, Shingo Yanagiya, Aika Miya, Hiraku Kameda, Kyu Yong Cho, So Nagai, Ichiro Sakuma, Akinobu Nakamura, Tatsuya Atsumi, on Behalf of the PARM-TD Study Group
- Received October 15, 2023 Accepted November 22, 2023 Published online February 29, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0370 [Epub ahead of print]
- 762 View
- 132 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Fibrates have renal toxicity limiting their use in subjects with chronic kidney disease (CKD). However, pemafibrate has fewer toxic effects on renal function. In the present analysis, we evaluated the effects of pemafibrate on the renal function of diabetic subjects with or without CKD in a real-world clinical setting.
Methods
We performed a sub-analysis of data collected during a multi-center, prospective, observational study of the effects of pemafibrate on lipid metabolism in subjects with type 2 diabetes mellitus complicated by hypertriglyceridemia (the PARM-T2D study). The participants were allocated to add pemafibrate to their existing regimen (ADD-ON), switch from their existing fibrate to pemafibrate (SWITCH), or continue conventional therapy (CTRL). The changes in estimated glomerular filtration rate (eGFR) over 52 weeks were compared among these groups as well as among subgroups created according to CKD status.
Results
Data for 520 participants (ADD-ON, n=166; SWITCH, n=96; CTRL, n=258) were analyzed. Of them, 56.7% had CKD. The eGFR increased only in the SWITCH group, and this trend was also present in the CKD subgroup (P<0.001). On the other hand, eGFR was not affected by switching in participants with severe renal dysfunction (G3b or G4) and/or macroalbuminuria. Multivariate analysis showed that being older and a switch from fenofibrate were associated with elevation in eGFR (both P<0.05).
Conclusion
A switch to pemafibrate may be associated with an elevation in eGFR, but to a lesser extent in patients with poor renal function.
- Drug/Regimen
- Switching to Once-Daily Insulin Degludec/Insulin Aspart from Basal Insulin Improves Postprandial Glycemia in Patients with Type 2 Diabetes Mellitus: Randomized Controlled Trial
- Kyu Yong Cho, Akinobu Nakamura, Chiho Oba-Yamamoto, Kazuhisa Tsuchida, Shingo Yanagiya, Naoki Manda, Yoshio Kurihara, Shin Aoki, Tatsuya Atsumi, Hideaki Miyoshi
- Diabetes Metab J. 2020;44(4):532-541. Published online November 22, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0093
- 5,618 View
- 157 Download
- 7 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background To explore the efficacy and safety of switching from once-daily basal insulin therapy to once-daily pre-meal injection insulin degludec/insulin aspart (IDegAsp) with respect to the glycemic control of participants with type 2 diabetes mellitus (T2DM).
Methods In this multicenter, open-label, prospective, randomized, parallel-group comparison trial, participants on basal insulin therapy were switched to IDegAsp (IDegAsp group;
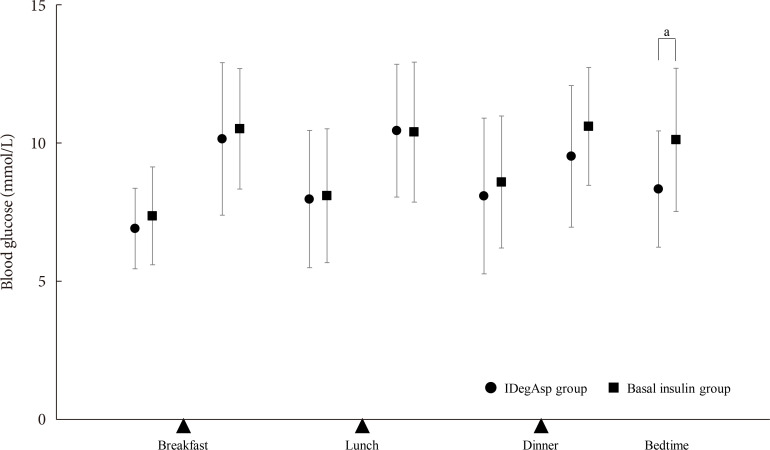
n =30) or continued basal insulin (Basal group;n =29). The primary endpoint was the superiority of IDegAsp in causing changes in the daily blood glucose profile, especially post-prandial blood glucose concentration after 12 weeks.Results Blood glucose concentrations after dinner and before bedtime were lower in the IDegAsp group, and the improvement in blood glucose before bedtime was significantly greater in the IDegAsp group than in the Basal group at 12 weeks (−1.7±3.0 mmol/L vs. 0.3±2.1 mmol/L,
P <0.05). Intriguingly, glycemic control after breakfast was not improved by IDegAsp injection before breakfast, in contrast to the favorable effect of injection before dinner on blood glucose after dinner. Glycosylated hemoglobin significantly decreased only in the IDegAsp group (58 to 55 mmol/mol,P <0.05). Changes in daily insulin dose, body mass, and recorded adverse effects, including hypoglycemia, were comparable between groups.Conclusion IDegAsp was more effective than basal insulin at reducing blood glucose after dinner and before bedtime, but did not increase the incidence of hypoglycemia. Switching from basal insulin to IDegAsp does not increase the burden on the patient and positively impacts glycemic control in patients with T2DM.
-
Citations
Citations to this article as recorded by- Glycaemic outcomes in hospital with IDegAsp versus BIAsp30 premixed insulins
Joshua R. Walt, Julie Loughran, Spiros Fourlanos, Rahul D. Barmanray, Jasmine Zhu, Suresh Varadarajan, Mervyn Kyi
Internal Medicine Journal.2024;[Epub] CrossRef - Low fasting glucose‐to‐estimated average glucose ratio was associated with superior response to insulin degludec/aspart compared with basal insulin in patients with type 2 diabetes
Han Na Jang, Ye Seul Yang, Tae Jung Oh, Bo Kyung Koo, Seong Ok Lee, Kyong Soo Park, Hak Chul Jang, Hye Seung Jung
Journal of Diabetes Investigation.2022; 13(1): 85. CrossRef - Comparing time to intensification between insulin degludec/insulin aspart and insulin glargine: A single-center experience from India
Rajiv Kovil
Journal of Diabetology.2022; 13(2): 171. CrossRef - Use of Insulin Degludec/Insulin Aspart in the Management of Diabetes Mellitus: Expert Panel Recommendations on Appropriate Practice Patterns
Tevfik Demir, Serap Turan, Kursad Unluhizarci, Oya Topaloglu, Tufan Tukek, Dilek Gogas Yavuz
Frontiers in Endocrinology.2021;[Epub] CrossRef - Pharmacoeconomic comparison of the second generation insulin analogs and insulins on their base
I. N. Dyakov, S. K. Zyryanov
Kachestvennaya Klinicheskaya Praktika = Good Clinical Practice.2021; 20(1): 4. CrossRef - Efficacy and Safety of Insulin Degludec/Insulin Aspart Compared with a Conventional Premixed Insulin or Basal Insulin: A Meta-Analysis
Shinje Moon, Hye-Soo Chung, Yoon-Jung Kim, Jae-Myung Yu, Woo-Ju Jeong, Jiwon Park, Chang-Myung Oh
Metabolites.2021; 11(9): 639. CrossRef - Insulin therapy in diabetic kidney disease
Yan Liu, Chanyue Zhao, Xiaofen Xiong, Ming Yang, Lin Sun
Diabetic Nephropathy.2021; 1(2): 67. CrossRef - Indirect comparison of efficacy and safety of insulin glargine/lixisenatide and insulin degludec/insulin aspart in type 2 diabetes patients not controlled on basal insulin
Anwar Ali Jammah
Primary Care Diabetes.2020;[Epub] CrossRef
- Glycaemic outcomes in hospital with IDegAsp versus BIAsp30 premixed insulins

 KDA
KDA
 First
First Prev
Prev





